Abstract
Background: Marginal zone lymphoma (MZL) is the third most common lymphoma in Western countries, accounting for 8-12% of non-Hodgkin lymphoma (Delinger NM. Cancer Manag Res. 2018;10:315-24). MZL is categorized into nodal, extranodal, and splenic subtypes. While the relative 5-year survival rate for patients (pts) with MZL is 84.4% (Olszewski AJ, Castillo JJ. Cancer. 2013;119:629-38), pts often experience relapse or refractory (R/R) disease. Parsaclisib is a potent, highly selective, next-generation inhibitor of phosphatidylinositol 3-kinase (PI3K)δ. Here we report results of the primary analysis of the cohort of Bruton's tyrosine kinase (BTK) inhibitor (BTKi)-naive pts with R/R MZL treated with parsaclisib monotherapy in the open-label, phase 2 study CITADEL-204 (NCT03144674, EudraCT 2017-000970-12).
Methods: Pts ≥18 years of age, with histologically confirmed MZL, prior receipt of ≥1 line of systemic therapy including anti-CD20 treatment, and either disease progression or inadequate response to most recent regimen were eligible. Pts had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≤2, radiologically measurable lymphadenopathy, lymphoid malignancy (extranodal), or histologically confirmed bone marrow infiltration (splenic). The study enrolled 2 cohorts: pts who received prior treatment with ibrutinib (cohort 1, N=10) or pts naive to BTKi therapy (cohort 2, N=100). Only data from cohort 2 are presented in this abstract. Pts were allocated to receive parsaclisib 20 mg once daily (QD) for 8 weeks followed by either 20 mg once weekly (weekly-dosing group [WG]) or 2.5 mg QD (daily-dosing group [DG]). Pts received prophylaxis for Pneumocystis jirovecii pneumonia during the study. The primary endpoint was objective response rate (ORR) as assessed by an independent review committee (IRC); secondary endpoints were duration of response (DOR), complete response rate (CRR), progression-free survival (PFS), overall survival (OS), and safety and tolerability. All radiology-based endpoints were evaluated by an IRC.
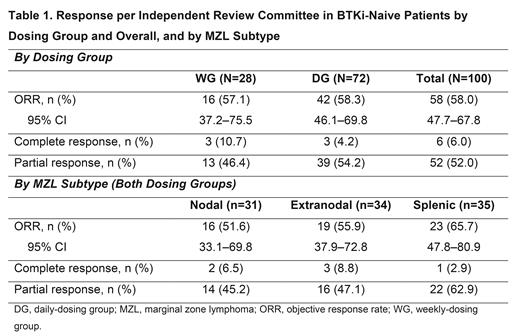
Results: At data cutoff for the primary analysis (Jan 15, 2021), 100 pts with MZL enrolled in cohort 2 (nodal 31.0%, extranodal 34.0%, and splenic 35.0%) had been treated (WG, N=28; DG, N=72). Median (range) age was 71.0 (35-95) years, 53.0% were male, and 95.0% had ECOG PS ≤1. 49.0% of pts had received 1 line, 31.0% received 2 lines, and 20.0% received ≥3 lines of prior systemic therapy (median [range], 2.0 [1-8]); 46.0% of pts had relapsed disease and 49.0% were refractory to their most recent prior therapy. 65.0% of pts had discontinued treatment, primarily due to adverse events (29.0%) or progressive disease (28.0%). The median (range) duration of treatment and follow-up from first dose to data cutoff were 13.4 (0.4-30.9) and 22.8 (11.9-37.0) months for all pts (N=100), and 11.6 (0.4-30.9) and 21.0 (11.9-37.0) months for the DG (N=72), respectively. The ORR (95% CI) was 58.0% (47.7-67.8) for all pts and 58.3% (46.1-69.8) for the DG (Table 1); CRR (95% CI) was 6.0% (2.2-12.6) for all pts and 4.2% (0.9-11.7) for the DG. Among all treated pts who achieved complete or partial response, 65.5% of responses occurred at the first disease assessment (median time to response, 8.1 weeks); median DOR (95% CI) was 12.2 (8.1-17.5) months for all pts and the DG. Median PFS (95% CI) was 16.5 (13.5-19.6) and 16.5 (11.5-20.6) months for all pts and the DG, respectively. Median OS was not reached.
Among 100 pts treated in cohort 2, treatment-emergent adverse events (TEAEs) occurred in 96.0% of pts (grade ≥3 in 63.0%). The most common TEAEs were diarrhea (47.0%) and cough (23.0%), and the most common grade ≥3 TEAEs included diarrhea (12.0%), neutropenia and pneumonia (9.0% each), and colitis (7.0%). TEAEs leading to dose interruption or dose reduction occurred in 56.0% and 16.0% of pts, respectively. TEAEs led to treatment discontinuation in 29.0% of pts, the most common were diarrhea (9.0%) and colitis (5.0%). Serious TEAEs were experienced by 47.0% of pts overall, the most common was pneumonia (9.0%). 6.0% of pts experienced fatal TEAEs including febrile neutropenia and sepsis (1 pt each) that were considered treatment-related.
Conclusion: Pts with R/R MZL demonstrated a rapid and durable clinical response to parsaclisib monotherapy. Treatment was generally well tolerated, and the safety profile was manageable. Results suggest parsaclisib may be a potential treatment option for R/R MZL.
Phillips: AstraZeneca: Consultancy; ADCT, BeiGene, Bristol Myers Squibb, Cardinal Health, Incyte, Karyopharm, Morphosys, Pharmacyclics, Seattle Genetics: Consultancy; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Consultancy, Research Funding; Bayer: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Incyte: Consultancy, Other: received travel expenses from Incyte, Research Funding. Avigdor: Pfizer: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; BMS: Research Funding; Janssen: Research Funding; Takeda: Consultancy, Honoraria. Gurion: JC Health CARE; Roche: Honoraria; Medison; Gilead Sciences; Takeda Pharmaceuticals: Consultancy. Corradini: KiowaKirin; Incyte; Daiichi Sankyo; Janssen; F. Hoffman-La Roche; Kite; Servier: Consultancy; AbbVie, ADC Theraputics, Amgen, Celgene, Daiichi Sankyo, Gilead/Kite, GSK, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, Takeda: Honoraria; Amgen; Takeda; AbbVie: Consultancy, Honoraria, Other: Travel and accommodations; Novartis; Gilead; Celgene: Consultancy, Other: Travel and accommodations; BMS: Other: Travel and accommodation; Sanofi: Consultancy, Honoraria; Incyte: Consultancy; AbbVie, ADC Theraputics, Amgen, Celgene, Daiichi Sankyo, Gilead/Kite, GSK, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, Takeda: Consultancy; Novartis, Janssen, Celgene, BMS, Takeda, Gilead/Kite, Amgen, AbbVie: Other: travel and accomodations. Mehta: Seattle Genetics; Incyte; TG Therapeutics: Consultancy; Seattle Genetics; Incyte; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Affirmed; Kite/Gilead; Roche-Genetech; Celgene/BMS; Oncotartis; Innate Pharmaceuticals; Seattle Genetics; Incyte; Takeda; Fortyseven Inc/Gilead; TG Therapeutics; Merck; Juno Pharmaceuticals/Bristol Myers Squibb: Research Funding. Lossos: NIH grants: Research Funding; Verastem: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees; University of Miami: Current Employment; NCI: Research Funding; Stanford University: Patents & Royalties; Seattle Genetics: Consultancy. Zinzani: Janssen-Cilag: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ImmuneDesign: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics Inc: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; BeiGene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck Sharp & Dohme: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Kyowa Kirin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Thieblemont: Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Hospira: Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses , Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Bayer: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses . Jurczak: AbbVie, AstraZeneca, Bayer, BeiGene, Celtrion, Celgene, Debbiopharm, Epizyme, Incyte, Janssen, Loxo Oncology, Merck, Mei Pharma, Morphosys, Novo Nordisk, Roche, Sandoz, Takeda, TG Therapeutics, Pharmacyclics, Affirmed, Gilead Sciences, Nordic Nanovecto: Research Funding; AstraZeneca, BeiGene, Janssen, Loxo Oncology, Sandoz, Roche: Membership on an entity's Board of Directors or advisory committees; Maria Sklodowska-Curie National Research Institute of Oncology: Current Employment; Jagiellonian University: Ended employment in the past 24 months; European Medicines Agency, Sandoz-Novartis, Janssen China R&D, BeiGene, Epizyme, Acerta, AstraZeneca: Consultancy. Zheng: Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Rappold: Incyte: Current Employment, Current equity holder in publicly-traded company. Zhao: Incyte: Current Employment, Current equity holder in publicly-traded company. Johnson: Epizyme: Consultancy, Research Funding; Boehringer Ingelheim: Consultancy; Bristol-Myers: Honoraria; Celgene: Honoraria; Genmab: Honoraria; Incyte: Honoraria; Kite Pharma: Honoraria; Kymera: Honoraria; Morphosys: Honoraria; Novartis: Honoraria; Takeda: Honoraria; Oncimmune: Consultancy; Janssen: Consultancy.
Investigational PI3Kdelta inhibitor (parsaclisib) for patients with MZL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal